ABOUT LIFEVALVES
LifeValves develops, manufactures, and markets innovative medical products and services to the healthcare industry. Our products provide security and comfort, ensuring the best results for patients and medical staff every day.
The company established as a result of the experience of Medical Doctors in the field, identifying problems causing complications and managed to develop an innovative solution solving those complications. Some of the issues are infection to the patient, contact with bodily fluids to the medical staff, in which these years of pandemic there's a necessity for extreme safety.
Values
Affordability to end-users – LifeValves wishes to help as many patients as possible worldwide, with emphasis on underprivileged populations in developing countries. Hence the company is attempting to create affordable products for the benefit of its day-to-day users;
Safety and comfort – LifeValves hopes to ease the patient's experience and reduce medical complications. The company's development is design to prevent collateral damages such as infections, injuries, etc. caused by catheterization;
Simplicity – LifeValves believes in simplifying catheterization related processes. Therefore, its solution is intuitive and easy to use, either by doctors or other caregivers;
Top medical standards – LifeValves believes in providing its customers with medical products with the highest standards of quality and professionalism.
Innovations in Medicines

OUR TECHNOLOGY
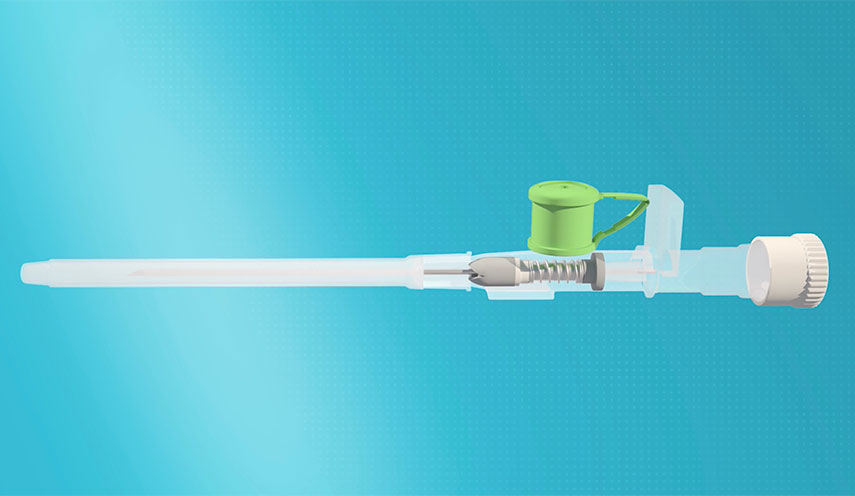
LifeValves has developed and patented a Catheter with an Internal Bi-Valve Security System. The catheter's main functionality is to prevent the backflow of fluids in the opposite direction from the purpose of the particular catheter in use.
The Bi-valvular security system is designed to adapt into any catheter system available in the market implemented in the use of extraction (or insertion) of biological substances such as IV peripheral catheters, arterial lines, urinary catheters, central lines, dialysis catheters, ventricular catheters, and biopsy catheters (oncology). The valve can be adjustable to any catheter without having to change the physical appearance of the catheter, for which it is intended to adapt the Bi-valvular system to the drainage funnel of the catheter in use. The Bi-valvular method consists of a sliding mechanism that can be applied to the simplest catheter and allows reinserting and extracting of the needle as done with any simple catheter.
While other valves exist in the market, the current device is unique by having a Bi-valvular system, causing it to be much more effective, cost-benefit for patients and hospitals, and better at preventing contaminations and complications.
Innovations in Medicines
OUR ADVANTAGE
Safety and reliability – Most importantly our medical products have to be reliable in terms of proven efficiency, non-harmful production materials, and safety mechanisms;
Our Competitive prices – The medical products industry as a whole is rich in the competition of great market influence. Furthermore, as a large portion of the market operates under an organized purchasing group (GPOs) or forms an integrated delivery network (IDNs), those organizations hold high negotiation leverages. Therefore, to succeed in owning a satisfactory market portion, constant observation and examination of competitors' pricing is required, as well as continually pushing prices down;
Positive branding – emphasizing the company's products as a better, healthier and safer choice compared to other current solutions is critical to enhancing consumers trust;
Easy to use – given that the company's products are to be used either by nurses and medical care providers or by patients themselves/ by their family members, the products must be intuitive to use and operate, and should not require specialized medical training;
The positioning of products – medical products must be positioned as 'Must-to-Have' to replace current leaders and be perceived as minimizing potential health-related risks.
Innovations in Medicines
OUR COMPETITIVE ADVANTAGE
First-mover advantage – LifeValves solution is virtually non-existent in today's market. LifeValves has a first-mover advantage of discovering a unique and beneficial backflow prevention method. With the same concept in mind, LifeValves will research and further develop its product line;
Strategic partners – LifeValves plans to operate in collaboration with an existing manufacturing company in the industry as an OEM/ODM (Original Equipment/Design Manufacturer) service. This collaboration will enable LifeValves manufacturing costs and require capital to be lower compared to other alternatives, as well as minimizing risks;
Proven efficiency and financial savings – The Bi-valvular security system will reduce costs per patient due to fewer complications and less of the medical staff's time spent per patient, in addition to less medical staff injuries;
Innovations in Medicines
OUR PHILOSOPHY

LifeValves develops, manufactures, and markets innovative medical products and services to the healthcare industry. Our products provide security and comfort, ensuring the best results for patients and medical staff every day.
The company established as a result of the experience of Medical Doctors in the field, identifying problems causing complications and managed to develop an innovative solution solving those complications. Some of the issues are infection to the patient, contact with bodily fluids to the medical staff, in which these years of pandemic there's a necessity for extreme safety.
Values
Affordability to end-users – LifeValves wishes to help as many patients as possible worldwide, with emphasis on underprivileged populations in developing countries. Hence the company is attempting to create affordable products for the benefit of its day-to-day users;
Safety and comfort – LifeValves hopes to ease the patient's experience and reduce medical complications. The company's development is design to prevent collateral damages such as infections, injuries, etc. caused by catheterization;
Simplicity – LifeValves believes in simplifying catheterization related processes. Therefore, its solution is intuitive and easy to use, either by doctors or other caregivers;
Top medical standards – LifeValves believes in providing its customers with medical products with the highest standards of quality and professionalism.
Innovations in Medicines
WHAT DO WE STAND FOR
Our vision is improving people's health around the world during their experiences with hospitals and medical needs by providing the best equipment to medical institutions. We create innovations in medical devices with sustainability in conjunction with medical providers providing a long-term solution and changing the medical devices for better and safer use.
Innovations in Medicines

OUR GOALS AND VISION
Introducing products that will represent an improvement to overall healthcare providers' experience;
Finish product development and ensure product manufacturing to comply with industry guidelines;
Continue to perform the necessary modifications to adjust the valve to all catheter types;
Achieve market awareness and establish a brand name so that LifeValves' products will perceive as safe
and preferable prevention for catheter-related possible complications.
Innovations in Medicines
OUR TEAM

Admiral Antonio (Tony) Zavala
Co-Founder & CEO
-
Read More
Antonio is a retired U.S. Army Master Sergeant, 1992-2010 (Chemical Specialist in Weapons of mass destruction), and Admiral of the Texas Navy. He served three tours of combat in Iraq (specialized in Weapons of mass destruction). Antonio was Master Sargent in the French Foreign Legion, Special Forces, 1985-1991, where he served six tours of combat in Africa.
Antonio holds numerous Academic degrees: M.S. in Homeland Security (Angelo State University, Angelo, TX), B.A. in Sociology, and B.A. in Business Administration (Washington State University, Pullman, WA) and B.A. in Homeland Security (American Military University, West Virginia).

Barak Blum
Co-Founder & Co-CEO
-
Read More
Barak is an Israeli veteran since 1982. Barak has many years of experience and volunteer work.
Barak has established and managed an institute for diagnosis and remedial instruction for learning and behavioral disabilities;
Chairman of IDF brain injury disabled veterans of the Israel IDF Disabled Veterans Organization;
Chairman of the Accessibility Committee at the Zichron Yaacov Local Council Steering Committee- "Accessible Moshav" Zichron Yaakov.
Barak has established a mutual assistance group of IDF brain injury disabled veterans;Barak is a partner in the establishment of "Yeladim" (Children) non-profit organization that
supports children and youth from all demographic sectors in Israel, who were placed in residential group homes and foster families;Medical photography at Belinson Hospital, Israel;
Private real estate investor and entrepreneur in the U.S;
Barak holds a B.A in Special Education from Tel Aviv University and has completed various courses such as holistic training (coaching) at Reidman College, fundamentals of Capital Markets course at Hi-Tech College, and an Advanced Technical analysis course at Meitav College.

Josef Brikman
Business Advisory Board
-
Read More
Josef is an accomplished executive with more than 28 years of experience in the company's management operation, P&L oversight, sales, multi-channel product distribution, and marketing involving both startups and established organizations, including a company listed at NASDAQ. Results-driven and decisive leader with a successful career in dynamic enterprises within domestic and international markets. Josef demonstrates high-caliber management qualifications, marketing instincts, experience-backed judgment, and excellent timing. Josef holds a Bachelor of Science in Engineering (Bs.c) from Fairleigh Dickenson University Teaneck, New Jersey Economics and Business Administration Haifa University Israel

Dr. Luis Fernando Servín de la Mora Godínez
Medical Team & Advisory Board
-
Read More
Dr. Luis Fernando is a Senior Neurosurgeon and Advanced Spine Surgeon at the Mexican Institute for Social Service IMSS Ensenada, Baja California, México
SUPPORTIVE QUALIFICATIONS
Experienced Neurosurgeon and Spine surgeon as well as primary care Physician in both Urban and Rural communities
Experience working with Specialized Surgeons, as well as with physicians specialized in other fields of medicine.
Reliable, good team worker, Respectful toward colleagues
Fluent in speech, reading and writing English (native level), Spanish (native), and Hebrew.
Member of the European Society for Spine Surgery EUROSPINE
EXPERIENCERegional General Hospital of Specialties No. 1 of the Mexican Institute of Social Service (IMSS) in Tijuana Baja California, Mexico, July/01/2003 to June/30/2004
Medical Internship Rotation (General Surgery, Subspecialty Surgery, Internal Medicine, Pediatrics, Family practice, Emergency Medicine, OB/GYN). With 100% hands-on training and a high level of patient exposure.
Rural Opportunities General Hospital No. 69 of the Mexican Institute of Social Service (IMSS) in San Quintin Valley, Ensenada, Baja California, Mexico, from August/01/2004 to July/31/2005.
Medical Social Service Attending Physician in Primary Care Medicine, General Consultation, and Obstetric Procedures. Assisting in General Surgery procedures and Gynecological Procedures.
Rural Emergency Medicine Attending Physician
Rural Community Medical Education Campaigns in Preventive Medicine Family Planification Campaigns.
Primary Care Physician for the State Public Health Sector, Tijuana, Baja California, Mexico. From February/2006 to January/2007.
Rural Population Health Brigades Attending Physician. From February/2007 to May/2007.
San Luis Rey General Hospital, Family Practice Attending Physician, Tijuana Baja California, Mexico. From May/2007 to January/2008.
Tel-Hashomer Medical Center, Dorothy Silverman Department of General Surgery (Under the Supervision of Professor Moshe Papa). General Surgery Training. From July/20/2008 to June/30/2009. Ramat-Gan, Israel.
Hadassah Medical Center Neurosurgical Department. Residency in Neurosurgery 2009- 2013.
Graduated from High Specialty Training in Hand Surgery, Micro-vascular, and Peripheral Nerve Reconstruction Surgery. Hadassah Medical Center Department of Plastic Surgery, Hand, Nerve, and Microsurgery Unit under the mentorship of Dr. Carol Pidhorz. 2012-2014
Attending Neurosurgeon, and Hand Surgery specialist, Hospital of the Mexican Red Cross, March 2014-November 2014
Intensive Fellow in Spine Surgery and Complex Spine Surgery, Rigshospitalet Department of Orthopedic Surgery, Spine Surgery Unit. Copenhagen, Denmark. 2015-2016.
Senior Neurosurgical Consultant for the Mexican Institute for Social Security Regional Hospital (IMSS), Obregon City, Sonora, Mexico 2016-2018
Senior Neurosurgeon and spine surgeon at IMSS, Ensenada, Baja California, Mexico. 2018- present.
Professor of Neurology and Neurosurgery at the Xochicalco University Ensenada campus 2019-present
MEDICAL EDUCATIONCentro de Estudios Universitarios Xochicalco, Escuela de Medicina (University of Xochicalco, School of Medicine). Tijuana, Baja California, Mexico. 1999-2005. University recognized and Listed in the WHO/IMED.
Degree as a Physician Surgeon Recognized by the Government of the State of Baja California and the Federal Gov. of Mexico (Secretary of Public Education and Ministry of Health).
Tel Hashomer Medical Center, the Dorothy Silverman Department of General Surgery. General Surgery Residency Training from July 20, 2008, to June 30, 2009. Ramat Gan, Israel. Tel Aviv University Sackler School of Medicine.
Hadassah Medical Center Department of Neurosurgery, Neurosurgery Residency Training 2009-2013. Ein Kerem, Jerusalem Israel. The Hebrew University of Jerusalem.
Hadassah Medical Center Department of Plastic Surgery, Hand Unit. High Specialty Residency Training in Hand Surgery, Microvascular, and Peripheral Nerve Reconstruction. Ein Kerem, Jerusalem Israel. 2012-2014. The Hebrew University of Jerusalem.
Microsurgery Skills Training Course, Department of Orthopedic Surgery Columbia University, New York City, USA. August 2012
IRCAD Training in Minimal Access Neurosurgery, Microscopic and Endoscopic Approaches to the Skull Base, Strasbourg France, January 2014. European Accreditation Council for Continuing Medical Education for Medical Specialist (EACCME)(UEMS), University of Strasbourg, France.
Intensive Fellowship in Spine Surgery, Complex Spine and Deformity Surgery, Orthopedic/Neurosurgical Combined. Rigshospitalet University Hospital, Department of Orthopedic Surgery, Spinal Surgery Unit. Under the supervision of Dr. Martin Gehrchen, Copenhagen, Denmark. 2015-2016
Neurosurgery Specialty Revalidation and Licensing by the General Director of Professions through the Office of Public Education and Health Professions (Ministry of Health) in Mexico
Up to Date Review Surgical Training in Complex Spine Surgery, Rigshospitalet Spine Surgery Unit, Department of Orthopedic Surgery, Copenhagen Denmark 22/01/2018- 26/01/2018.
PUBLICATIONS AND CONGRESS PRESENTATIONSThe Israeli Experience In the Management of Traumatic Carotid Dissections With Endovascular Means. Silan, Portugal 2011 Neurosurgical Congress.
Island Digital Flaps, Use, and Effectiveness, Servin, Pidhorz, The Israeli Congress for Surgery of the Hand 2012.
Flexor Carpi Radialis Tenosynovitis: Review of Literature and Personal Experience, L. Servin de la Mora G., Pidhorz in the 23rd Annual Meeting of the Israeli Society for Surgery of the Hand, November 2013. Final Work Presentation to obtain the Degree of Specialist in Hand Surgery under the Supervision of Dr. Carole Pidhorz.
Fluoroscopy Assistance in the placement of Ventriculoperitoneal Derivation System: Gerardo Antonio Reyes Jimenez MD, Luis Vicente Gomez Bañuelos MD, Luis Fernando Servin de la Mora Godínez MD*, Orlando Tortoledo-Barron MD. Unidad Médica de Alta Especialidad, Hospital Regional de Especialidades, Cd. Obregon Sonora, Mexico. 2017

Dr. Erick Alejandro Servín de la Mora Godínez
Medical Team & Advisory Board
-
Read More
Dr. Erick Alejandro graduated from the University of Xochicalco, School of Medicine, in 2004 as a surgeon. Dr. Erick Alejandro has over 14 years of medical experience in various areas. The following are some of his accomplishments, places of employment, and medical experience:
2005-2006 – Medical Intern at the General Hospital of Tijuana;
2006-2007 – Intern at Social Service SSA Guachochi Chihuahua;
2008-2012 – Specialty in Traumatology and Orthopedics at General Hospital of Querétaro, Querétaro, Qro;
2012-Present – Medical Specialist Traumatology and Orthopedics at IMSS Regional Hospital

Dr. Pedro Manuel Servín de la Mora Godínez REST IN PEACE
Medical Team & Advisory Board
-
Read More
Dr. Pedro Manuel graduated from the University of Xochicalco, School of Medicine in 2006 as a 'Medico Cirujano' (Surgeon Doctor, or General Physician). Dr. Pedro Manuel has over 12 years of medical experience in various areas. The following are some of his accomplishments, places of employment, and medical experience:
2006 (after graduating) – Clinical rotational internship at the ISSSTECALI Hospital in Tijuana;
2007 – Social service sector: Santa Rosalia General Hospital E.R., San Ignacio Health Center, and at Villarberto Alvarado Aramburo Health Center in Vizcaino;
2009-2013 – Private medical practice sector: International Biocare and Wellness center (IBC) in Tijuana as an emergency physician, Chipsa Medical Center in Playas de Tijuana as an on-call E.R. physician and as a general medical consultant, and Hospital General de Tecate in Tecate as an emergency room physician;
2013-2018 – Anesthesiology residency program at the Department of Anesthesiology, Intensive Care, and Pain Management under the supervision of Prof. Charles Wiessman, and Prof. Reuben Pizzof at Hadassah Medical Center in Ein Kerem, Jerusalem Israel.

Dr. Noemi Garcia Huesca
Surgeon, Genomic medicine, Medical Expert

Marta Ayllon
Industrial Engineer, COO Chief Operation Officer

Genevieve Zavala
Transmedia Communications, Business Development VP
THE MARKET
The catheter-related medical device market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle-East, and according to Grand View Research are the following insights:
North-America held the largest share in 2014 due to affordability of advanced devices because of well-structured reimbursement procedures and a large number of surgeries as a consequence of the prevalence of lifestyle-associated disorders;
The Asia Pacific is expected to be the fastest-growing region due to factors such as increased healthcare expenditure and the growing demand for technologically advanced products;
Emerging economies, such as China and India, show a growing demand for medical products. The purchasing power of the ever-increasing middle class is expected to increase to 42% by 2020 from 23% in 2009, so the patient population in emerging countries is increasingly opting for technologically advanced medical products, which drives demand for medical devices and catheterization processes.
End-User Characteristics.

Urinary incontinence increases with age: from 14% in ages 65–69 years to 45% in ages over 85 years. Therefore, the majority of users are elderly;
Due to its effects on quality of life, urinary incontinence patients with financial means seek residential care and ease to their discomfort;
Immobile patients are less suitable for alternative solutions (such as incontinence pads); therefore, they are more likely to use catheters.
(Above data is based on a PMC research from 2015)
Innovations in Medicines
Medical profile
The aging population is more susceptible to disorders like cardiac arrest, renal (kidney) failure, cirrhosis, and other conditions that require the use of catheters in their treatment. Therefore, they are expected to show an increasing need for catheters and related devices;
The growing prevalence of lifestyle-associated diseases like cardiovascular disorders, chronic renal failure, and a few types of cancer will affect the number of surgeries and, as a result, demand for catheters.
Sustainability
LifeValves commits with our philosophy as a company in long-standing sustainability; we constantly promote social responsibility in everything we do and other companies that have a relationship with us. We in our day-to-day assure that our commitment to sustainability is not temper; we commit to reducing our environmental footprint while delivering the best of products to our customers, taking into consideration the health of the individuals and the medical providers.
Safety in Healthcare
Probably the biggest challenge in these days of infections and diseases that medical professionals face today is the necessary safety and patient care maintaining efficiency while providing medical assistance. That is why LifeValves commits to assure that our products are safe for the medical providers as well as giving the latest state-of-the-art products for the patients ensuring their safety of any complications from the use of medical devices.
Compliance
LifeValves Limited is committed to the highest standards of ethical practice around the world.
LifeValves intends to be recognized for the best products and the service provided to the medical teams and patients around the world. We consistently conduct our business in an honest and ethical basis. We hold our commitment to the highest standards and firm moral beliefs and integrity, together with our policies and procedures. We are demonstrated by the excellence of our employees and their ethical behavior representing the highest in the business, providing the customer, suppliers, competitors, and governmental agencies the integrity that characterizes our company and compliance policies.
MISSION
WHO WE ARE
LifeValves is a company dedicated to providing the latest innovations for medical providers to maintain safety and simplicity in the use of our products.
EXPERIENCE
The lengthy experience of our team of medical professionals have manufacture products that will ease patient experience with medical personal and also reduce medical complications by simplifying medical procedures
OUR GOALS
Our guarantee to introduce the best products that will represent improvements to overall healthcare providers around the world, assuring that they have the best materials to serve their patients with safety and simplicity.
CONTACT

News and events
LifeValves is excited to announce that in March of 2020, the patent to our Bi-Valve was granted.
Get In Touch
Address:
LifeValves OÜ
Harju Maakond,
Tallinn, Kesklinna linnaosa,
Narva mnt 5, 10117 Estonia
Phone:
USA +1469-242-3539
Israel +97587393068
Spain +34 666931860
Email: information@lifevalves.com
Website: www.lifevalves.com
INVESTORS
for more information
please contact us:
USA +1469-242-3539
Israel +97587393068
Spain +34 666931860
Email: information@lifevalves.com
Website: www.lifevalves.com
WISMED® GROUP
LifeValves, a division of WisMed® Group
WISMED® – is where simplicity meets technology. Our mission is to put patients and healthcare providers first by delivering safety through our product. By accomplishing our mission, we create the highest value for our team, partners, and shareholders.



